Introduction
Sulfur compounds and mercaptans in hydrocarbon and petroleum cuts specially gas condensate, lead to environmental pollution and corrosion problems in pipelines and storage tanks. Thus, it is necessary to decrease sulfur and mercaptans.
What are mercaptans?
Mercaptans, commonly referred to as thiols, are organosulfur molecules composed of carbon, hydrogen, and sulfur that are known for having a pungent odor similar to rotten cabbage or garlic. Chemically they can be shown as “RSH” where R is a hydrocarbon that is attached to sulfur and hydrogen. “Methanethiol” for example is a mercaptan. The chemical structure of this molecule is shown here:
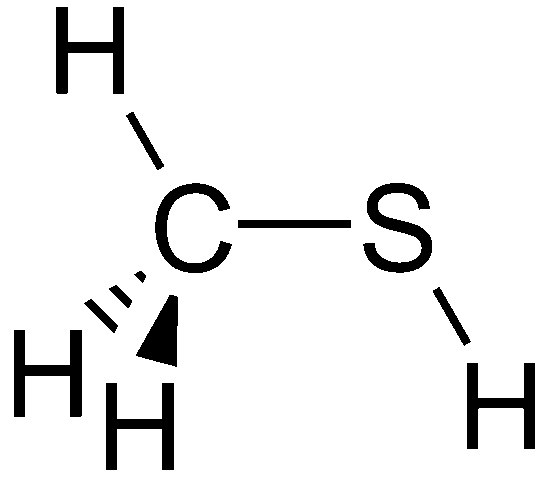
What are the methods for demercaptanization?
The following methods are used for demercaptanization process:
- Merox
Merox is an acronym for mercaptan oxidation. It is a proprietary catalytic chemical process developed by UOP used in oil refineries and natural gas processing plants to remove mercaptans from LPG, propane, butanes, light naphthas, kerosene and jet fuel by converting them to liquid hydrocarbon disulfides.
The Merox process requires an alkaline environment which, in some process versions, is provided by an aqueous solution of sodium hydroxide (NaOH), a strong base, commonly referred to as caustic. In other versions of the process, the alkalinity is provided by ammonia, which is a weak base.
The catalyst in some versions of the process is a water-soluble liquid. In other versions, the catalyst is impregnated onto charcoal granules.
Versions of the Merox process for various applications (based on UOP standards):
1.Conventional Merox for extraction of mercaptans from LPG, propane, butanes or light naphthas.
2.Conventional Merox for sweetening jet fuels and kerosenes.
3.Merox for extraction of mercaptans from refinery and natural gases.
4.Minalk Merox for sweetening of naphthas: This process continuously injects just a few ppm of caustic into the feed naphtha.
5.Caustic-free Merox for sweetening jet fuels and kerosenes: This process injects small amounts of ammonia and water (rather than caustic) into the feed naphtha to provide the required alkalinity.
6.Caustic-free Merox for sweetening of naphthas: This process also injects small amounts of ammonia and water (rather than caustic) into the feed naphtha to provide the required alkalinity.
In all of the Merox versions, the overall oxidation reaction that takes place in converting mercaptans to disulfides is:
4RSH + O2 → 2RSSR + 2H2O
- DMD/DMC (IVKAZ)
DMD process has been developed as a demercaptanization process providing the possibility of demercaptanization of different hydrocarbon cuts even crude oil as a feed.
Chemical reaction that happens in DMD process:
H2S+NaOH→Na2S+H2O
This technology consists of 3 steps:
1.Extraction of COS, D2S, CS2 and light mercaptans
2.Oxidation of heavy mercaptans
Heavy mercaptans in the feed, will not be separated by caustic, but they easily oxidize and turn into disulfide. The feed that consists of heavy mercaptans, should entered to oxygen rich (60% wt Air) demercaptanization reactor in presence of caustic solution and IVKAZ catalyst, so the oxidation reaction can occur.
3.Regeneration of caustic used in the extraction
In this process the caustic that used in extraction of light mercaptans in presence of air and IVKAZ will be oxidize and mercaptans in caustic turns into disulfide and separate from caustic.
Light mercaptan compounds (C1-C3), separate from the system using caustic solution (8-10%) in presence of IVKAZ catalyst.
RSH+NaOH→RSNa+H2O
DMC process
For the purpose of gas condensate demercaptanization, In the first stage of the process (Pre-wash) hydrogen sulfide, the main quantity of low molecular weight mercaptans C1-C2 and naphthenic acids are extracted by circulating alkaline solution. In the second stage (Extraction) removal of C2-C3 mercaptans and a part of C4 mercaptans is performed by using regenerated alkaline catalytic solution, which will be routed to the first stage of extraction afterwards. The third stage (Oxidation) of the process provides deep oxidative demercaptanization of feed in oxidation reactor in the presence of air, enriched with oxygen, and homogeneous IVKAZ catalyst. Mercaptans C4+, which could not be removed during extraction stages, will be converted to non-toxic dialkyl disulfides in the oxidation stage, and remain in treated condensate.
The regeneration (Caustic Regeneration Stage) of the alkaline catalyst complex solution (CC) is carried out by catalytic oxidation of sodium sulfide and mercaptides in the presence air oxygen over homogeneous IVKAZ catalyst. Dialkyl disulfides, formed during mercaptide oxidation, dissolve in the condensate afterwards.
Compared to other methods, while being more economical, this process has shown better results in the purification of gas condensate and also purifies more compounds. A large part of South Pars gas condensate is purified by this method and in addition to eliminating the unpleasant smell of the condensate, the amount of mercaptan and hydrogen sulfide reaches less than 50 ppm.
Chemistry of DMC Process
Stage 1 and 2: Extraction of hydrogen sulfide and mercaptans C1-C3
Chemistry of condensate treatment for hydrogen sulfide and light mercaptans C1-C3 is based on their extraction by using aqueous sodium hydroxide solution in the form of mercaptides and sodium sulfide as the following reactions:
H2S + 2NaOH→Na2S + 2H2O [1]
RCOOH + NaOH→RCOONa + H2O [2]
RSH + NaOH↔RSNa + H2O [3]
Three reactions proceed at high excess rate of sodium hydroxide at a temperature of 20-70 °C. Reactions 1 and 2 are irreversible and exothermic. Reaction 3 is reversible and exothermic and at a temperature higher than 90 °C the reaction equilibrium shifts to the left, i.e. to the side of mercaptan formation.
Stage 3: Oxidation of mercaptans C4+
Heavy mercaptans (C4+) are oxidized over homogeneous phthalocyanine catalyst IVKAZ with oxygen of air to form disulfides by the following reaction at a temperature of 50-70 °C:
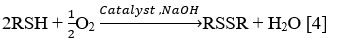
Stage 4: Regeneration stage of the caustic solution
Regeneration of alkaline solution, containing sodium mercaptides and sulfide, proceed over homogeneous catalyst solution IVKAZ by oxidizing sodium sulfide and sodium mercaptides with oxygen of air by the following reactions:
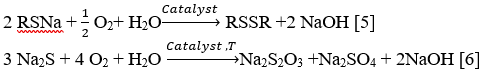
The reactions are catalytic, exothermic and are accelerated by increasing temperature, pressure, air and catalyst quantities.
ODS process
The oxidative desulfurization (ODS) process is a method for deep desulfurization of fuels under mild reaction conditions. In this process, S-compounds are oxidized to their corresponding sulfones/sulfoxides using a catalyst in the presence of a suitable oxidant. The oxidized S-compounds from fuel oil have more polarity, boiling temperature, and molecular weight that facilitate their separation by different approaches such as extraction, adsorption, etc. The ODS process involves two steps: oxidation, followed by separation. Oxidants, catalysts, and operating conditions play an important role in the ODS process. Oxidants donate an oxygen atom to the sulfur compounds in BT (benzothiophene), DBT (dibenzothiophene), and their derivatives to form sulfones or sulfoxides under mild conditions. Among different oxidants, H2O2 has shown the best performance because it is inexpensive, environment friendly, commercial available, and contains the highest percentage of active oxygen. The second step in the ODS process is the separation from liquid fuel on the oxidized compounds.
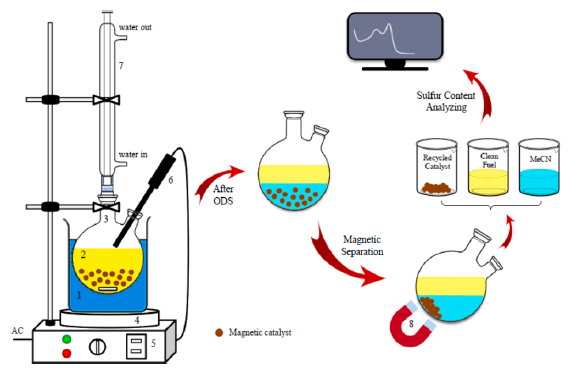
POM
POMs have been frequently applied as catalysts in the ODS process because of their condensable properties, including mild reaction conditions, hydrolytic and thermal stability, high selectivity, and tunable catalytic properties. POMs have been applied as either homogeneous or heterogeneous methods for the ODS of fuels. But they also have relatively weak interaction such as adsorption or electrostatic interactions.
The advantages and problems of ODS
Advantages:
- High performance
- Process simplification by combination of extraction
- Effectiveness in obtaining ultralow levels of S-compounds.
Problems:
- Residual sulfones treatment obtained from S-compounds oxidation.
- POMs (type of catalysts used in ODS) have relatively weak interaction such as adsorption or electrostatic interactions.
HDS process
Hydrodesulfurization (HDS) is the process that organosulfur compounds are removed over heterogenous catalysts during the refinement of petroleum. HDS is effective to achieve ultralow sulfur levels in the products. However, challenges associated with this method are:
- High hydrogen consumption
- Difficult operating conditions
- Low removal efficiency of compounds such as BT, DBT, and their derivatives.
Mercaptan scavengers
Mercaptan scavengers are liquid additives that react irreversibly with all species of mercaptan sulfur to form oil-soluble, heat-stable products in crude oils, asphalt, residual fuels, and refined fuels.
Mercaptan removal methods in general
One option, extraction, dissolves the disulfides in caustic and removes them. The other option, sweetening, leaves the converted disulfides in the product. Extraction removes sulfur, sweetening just removes the mercaptan odor.